First observation of rubber-like elasticity in liquid glycerol
Simple molecular liquids such as water or glycerol are of great importance for technical applications, in biology or even for understanding properties in the liquid state. Researchers at the MPSD have now succeeded in observing liquid glycerol in a completely unexpected rubbery state. Writing in PNAS, they report how they created rapidly expanding bubbles on the surface of the liquid in vacuum using a pulsed laser. However, the thin, micrometers-thick liquid envelope of the bubble did not behave like a viscous liquid dissipating deformation energy as expected, but like the elastic envelope of a rubber toy balloon, which can store and release elastic energy.
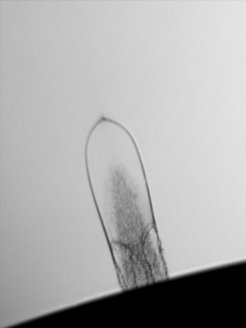
It is the first time an elasticity dominating the flow behavior in a Newtonian liquid like glycerol has been observed. Its existence is difficult to reconcile with common ideas about the interactions in liquid glycerol and motivates the search for more comprehensive descriptions. Surprisingly, the elasticity persists over such long timescales of several microseconds that it could be important for very rapid engineering applications such as micrometer-confined flows under high pressure. Yet, the question remains unsettled whether this behavior is a specific property of liquid glycerol, or rather a phenomenon that occurs in many molecular liquids under similar conditions but has not been observed so far.
The team proposes that the high straining rate and the confined thickness of the shell causes the individual molecules to form groups that are displaced in a correlated and collective manner. This change would stabilize the elastic state over a longer time than would be possible in glycerol’s equilibrium state, where the single molecules are subject to fast diffusion. “We want to reach a better understanding of this unusual state,” says lead author and doctoral student Meghanad Kayanattil, “because it could tell us a lot about collective excitations in disordered systems.”
The existence of such a rubber-like state in liquid glycerol raises the question: Are similar effects possible in other liquid substances? In particular the creation of elastic bubbles in water would be a major achievement because it is the most important and well-studied liquid with implications for multiple scientific fields. However, the glycerol bubbles only formed in a vacuum environment, as shown by the MPSD team. This poses some challenges for similar experiments involving water, because it begins to boil below the vapor pressure of 32 mbar – well above the pressure at which the experiments need to take place.
The research was carried out by members of the Institute’s Scientific Support Unit Ultrafast Beams and guest scientist Zhipeng Huang from the University of Duisburg-Essen. An innovative scientific approach and the right choice of parameters led to the discovery of this novel elastic behavior. “Our experiment invites us to rethink the correlations and the differences between liquids and solids,” says principal investigator Sascha Epp. “As a next step, we aim to investigate the molecular interaction and structure of the transient bubble shell and whether this effect can also be created in a range of other liquids whose molecular interactions are different from glycerol.”












